Photophysical properties of pyrrolocytosine, a cytosine fluorescent base analogue†
Abstract
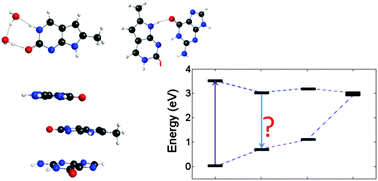
The photophysical behavior of pyrrolocytosine (PC), a fluorescent base analogue of cytosine, has been investigated using theoretical approaches. The similarities between the PC and cytosine structures allow PC to maintain the pseudo-Watson–Crick base-pairing arrangement with guanine. Cytosine, similar to the other natural nucleobases, is practically non-fluorescent, because of ultrafast radiationless decay occurring through conical intersections. PC displays a much higher fluorescence quantum yield than cytosine, making it an effective fluorescent marker to study the structure, function, and dynamics of DNA/RNA complexes. Similar to 2-aminopurine, a constitutional isomer of adenine that base-pairs with thymine, PC's fluorescence is quenched when it is incorporated into a dinucleotide or a trinucleotide. In this work we examine the photophysical properties of isolated PC, microhydrated PC, as well as, complexes where PC is either base-stacked or hydrogen-bonded with guanine. Our results indicate that hydration affects the radiationless decay pathways in PC by destabilizing conical intersections. The calculations of dimers and trimers show that the radiative decay is affected by π stacking, while the presence of charge transfer states between PC and guanine may contribute to radiationless decay.
- This article is part of the themed collection: Prebiotic chemistry and the molecular origins of life

 Please wait while we load your content...
Please wait while we load your content...