Deuteron quadrupole coupling constants and reorientational correlation times in protic ionic liquids†
Abstract
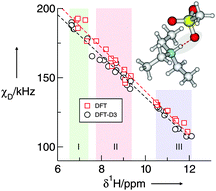
We describe a method for the accurate determination of deuteron quadrupole coupling constants χD for N–D bonds in triethylammonium-based protic ionic liquids (PILs). This approach was first introduced by Wendt and Farrar for O–D bonds in molecular liquids, and is based on the linear relationship between the deuteron quadrupole coupling constants χD, and the proton chemical shifts δ1H, as obtained from DFT calculated properties in differently sized clusters of the compounds. Thus the measurement of δ1H provides an accurate estimate for χD, which can then be used for deriving reorientational correlation-times τND, by means of NMR deuteron quadrupole relaxation time measurements. The method is applied to pure PILs including differently strong interacting anions. The obtained χD values vary between 152 and 204 kHz, depending on the cation–anion interaction strength, intensified by H-bonding. We find that considering dispersion corrections in the DFT-calculations leads to only slightly decreasing χD values. The determined reorientational correlation times indicate that the extreme narrowing condition is fulfilled for these PILs. The τc values along with the measured viscosities provide an estimate for the volume/size of the clusters present in solution. In addition, the correlation times τc, and the H-bonded aggregates were also characterized by molecular dynamics (MD) simulations.

 Please wait while we load your content...
Please wait while we load your content...