A comprehensive picture of the ultrafast excited-state dynamics of retinal†
Abstract
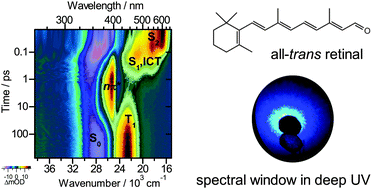
All-trans retinal is the chromophore of microbial rhodopsins initiating energy conversion and cellular signalling by subpicosecond photoinduced switching. Here, we provide detailed UV-Vis transient absorption experiments to disentangle the complex photochemistry of this polyene, which is governed by its terminal aldehyde group. After photoexcitation to the S2(1Bu+) state, the system exhibits polarity-dependent branching, populating separate S1(1Ag−) and intramolecular charge transfer (ICT) species. In all solvents, population of a singlet nπ* state from S1 is observed which represents the precursor of the T1 triplet state. While triplet formation dominates in nonpolar solvents (67% quantum yield), it is dramatically reduced in polar solvents (4%). The channel closes completely upon replacing the aldehyde by a carboxyl group, due to an energetic up-shift of 1nπ*. In that case, internal conversion via the ICT species becomes the main pathway, with preferential formation of the initially excited isomer.

 Please wait while we load your content...
Please wait while we load your content...