Photoluminescence properties of Yb2+ ions doped in the perovskites CsCaX3 and CsSrX3 (X = Cl, Br, and I) – a comparative study†
Abstract
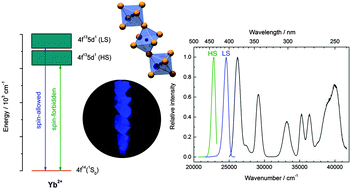
The Yb2+-doped perovskite derivatives CsMX3 (M = Ca and Sr; X = Cl, Br, and I) are ideal systems for obtaining a detailed insight into the structure–luminescence relationship of divalent lanthanides. The investigation of the respective photoluminescence properties yielded two emission bands in the violet and blue spectral range for all compounds, which are assigned to the spin-allowed and spin-forbidden 5d–4f transitions, respectively. The impact on their energetic positions is dependent on both the covalency of the Yb2+-halide bond and the corresponding bond length in agreement with expectations. The excitation spectra provide a detailed fine structure at low temperatures and can be partly interpreted separating the 4f13 core from the 5d electron in the excited state. The local crystal field in CsSrI3:Yb2+ provides a special case due to the trigonal distortion induced by the crystal structure that is clearly evident in the luminescence features of Yb2+. The structure–property relationship of several spectroscopic key quantities of Yb2+ in this series of halides is analyzed in detail and parallels the properties of Eu2+ ions doped in the given perovskites.

 Please wait while we load your content...
Please wait while we load your content...