Molecular degradation of D35 and K77 sensitizers when exposed to temperatures exceeding 100 °C investigated by photoelectron spectroscopy†
Abstract
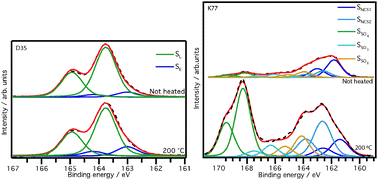
Degradation of the materials in dye-sensitized solar cells at elevated temperatures is critical for use in real applications. Both during fabrication of the solar cell and under real working conditions the solar cells will be exposed to heat. In this work, mesoporous TiO2 electrodes sensitized with the dyes D35 and K77 were subject to heat-treatment and the effects of this were thereafter investigated by photoelectron spectroscopy. For D35 it was found that heat-treatment changes the binding configuration inducing an increased interaction between the sulfur of the linker unit and the TiO2 surface. The interaction resulting from the change in binding configuration also affects the position of the HOMO level, where a shift of +0.2 eV is observed when heated to 200 °C. For K77, parts of the thiocyanate units are detached and the nitrogen atom leaves the electrode whereas sulfur remains on the surface in various forms of sulfurous oxides. The total dye coverage of K77 gets reduced by heat-treatment. The HOMO level gets progressively less pronounced due to a loss of HOMO level electrons as a consequence of the lower dye coverage when heat-treated, which leads to a lower excitation rate and lower efficiency. The results are discussed in the context of performance for dye-sensitized solar cells.

 Please wait while we load your content...
Please wait while we load your content...