Aggregation-induced emission of diarylamino-π-carborane triads: effects of charge transfer and π-conjugation†
Abstract
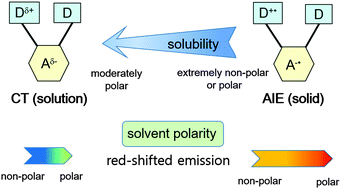
Carborane-based donor–π–acceptor triads (D–π–A–π–D) bearing triarylamine moieties were synthesised. All the monomeric triads showed a blue-green emission in a dilute solution, which was assigned as an intramolecular charge-transfer (CT) emission. The intramolecular CT emission showed large Stokes shifts at a higher solvent polarity. The intramolecular CT emission further shifted to a longer wavelength with the increase in π-conjugation. Interestingly, a strong red emission was observed in highly concentrated solutions or in the solid state, which was assigned as an aggregation-induced emission (AIE). Moreover, the AIE strongly depended on solvent polarity. A large Stokes shift in AIE was attributed to the strong CT character. The changes in the dipole moment for the AIE state and monomer emission were evaluated using the Lippert–Mataga relationship. The density functional theory calculations showed that the change in electron distribution between the aryl amino group (highest occupied molecular orbital, HOMO) and the carborane moiety (lowest unoccupied molecular orbital, LUMO) indicates the intramolecular CT character, and the emission colour changes were attributed to the HOMO–LUMO energy gap controlled by the π-extension of the phenylene linker. The electrochemical properties such as oxidation and reduction potentials were consistent with theoretical calculation results. The emission properties were affected by two main factors: solvent polarity and solubility.

 Please wait while we load your content...
Please wait while we load your content...