Effect of lipid head group interactions on membrane properties and membrane-induced cationic β-hairpin folding†
Abstract
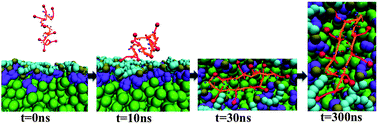
Stages in POPS membrane induced SVS-1 folding. One key characteristic of mIFs is the dielectric gradient and subsequently, electrostatic potential that arises from dipolar interactions in the head group region. In this work, we present a coarse-grained (CG) model for anionic and zwitterionic lipids that accounts for dipolar intricacies in the head group region. Prior work on adding dipolar interactions in a coarse grained (CG) model for peptides enabled us to achieve α/β secondary structure content de novo, without any added bias. We have now extended this idea to lipids. To mimic dipolar interactions, two dummy particles with opposite charges are added to CG polar beads. These two dummy charges represent a fluctuating dipole that introduces structural polarization into the head group region. We have used POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine) and POPS (1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine) as our model lipids. We characterize structural, dynamic, and dielectric properties of our CG bilayer, along with the effect of monovalent ions. We observe head group dipoles to play a significant role in membrane dielectric gradient and lipid clustering induced by dipole–dipole interactions in POPS lipids. In addition, we studied membrane-induced peptide folding of a cationic antimicrobial peptide with anticancer activity, SVS-1. We find that membrane-induced peptide folding is driven by both (a) cooperativity in peptide self-interaction and (b) cooperativity in membrane–peptide interaction. In particular, dipolar interactions between the peptide backbone and lipid head groups contribute to stabilizing folded conformations.

 Please wait while we load your content...
Please wait while we load your content...