The mechanism of excited state proton dissociation in microhydrated hydroxylamine clusters†
Abstract
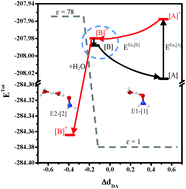
The dynamics and mechanism of excited-state proton dissociation and transfer in microhydrated hydroxylamine clusters are studied using NH2OH(H2O)n (n = 1–4) as model systems and the DFT/B3LYP/aug-cc-pVDZ and TD-DFT/B3LYP/aug-cc-pVDZ methods as model calculations. This investigation is based on the Förster acidity scheme and emphasizes the photoacid dissociation in the ground (S0) and lowest singlet-excited states (S1) and the interplay between the photo and thermal excitations. The quantum chemical results suggest that the intermediate complexes are formed only in the S1 state in a low local-dielectric environment (e.g., ε = 1) and that upon the S0 → S1 transition, the photon energy excites mostly NH2OH, which leads to a homolytic cleavage of the O–H bond and to dynamically stable charge-separated Rydberg-like H-bond complexes (e.g., NH2O˙–H3O+˙). The potential energy surfaces for proton displacement in the smallest Rydberg-like H-bond complex support the intersection of the S0 and S1 states in low local-dielectric environments, whereas in a high local-dielectric environment (e.g., ε = 78), these two states are completely separated. Based on the static results, a photoacid-dissociation mechanism that involves Rydberg-like H-bond complex formation, an H-bond chain extension and fluctuations in the local-dielectric environment is proposed. NVT-BOMD simulations confirm the static results and show that the dynamic behavior of the dissociating proton in the S1 state is not different from that of the protonated H-bond systems in the ground state, which consists of the oscillatory shuttling and structural diffusion motions. These findings allow our theoretical methods, which have been used successfully in protonated H-bond systems in the ground state, to be applied in the study of the photoacid-dissociation processes. The current theoretical study suggests effective steps as well as guidelines for the investigation of the dynamics of the photoacid-dissociation and transfer processes in the Förster acidity scheme, provided that the exciting photon does not lead to a significant change in the structure of the intermediate complex in the excited state.

 Please wait while we load your content...
Please wait while we load your content...