The role of hydrogen during Pt–Ga nanocatalyst formation†
Abstract
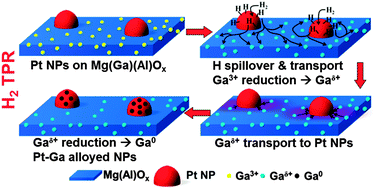
Hydrogen plays an essential role during the in situ assembly of tailored catalytic materials, and serves as key ingredient in multifarious chemical reactions promoted by these catalysts. Despite intensive debate for several decades, the existence and nature of hydrogen-involved mechanisms – such as hydrogen-spillover, surface migration – have not been unambiguously proven and elucidated up to date. Here, Pt–Ga alloy formation is used as a probe reaction to study the behavior and atomic transport of H and Ga, starting from Pt nanoparticles on hydrotalcite-derived Mg(Ga)(Al)Ox supports. In situ XANES spectroscopy, time-resolved TAP kinetic experiments, HAADF-STEM imaging and EDX mapping are combined to probe Pt, Ga and H in a series of H2 reduction experiments up to 650 °C. Mg(Ga)(Al)Ox by itself dissociates hydrogen, but these dissociated hydrogen species do not induce significant reduction of Ga3+ cations in the support. Only in the presence of Pt, partial reduction of Ga3+ into Gaδ+ is observed, suggesting that different reaction mechanisms dominate for Pt- and Mg(Ga)(Al)Ox-dissociated hydrogen species. This partial reduction of Ga3+ is made possible by Pt-dissociated H species which spillover onto non-reducible Mg(Al)Ox or partially reducible Mg(Ga)(Al)Ox and undergo long-range transport over the support surface. Moderately mobile Gaδ+Ox migrates towards Pt clusters, where Gaδ+ is only fully reduced to Ga0 on condition of immediate stabilization inside Pt–Ga alloyed nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...