Two reaction regimes in the oxidation of larger cationic tantalum clusters (Tan+, n = 13–40) under multi-collision conditions†
Abstract
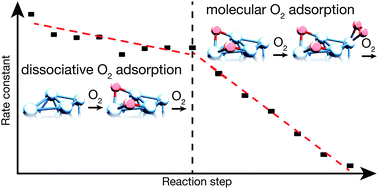
The reaction of cationic tantalum clusters (Tan+, n = 13–40) with molecular oxygen is studied under multi-collision conditions and at different temperatures. Consecutive reaction proceeds in several steps upon subsequent attachment of O2. All cluster sizes exhibit fast reaction with oxygen and form a characteristic final reaction product. The time-dependent product analysis enables the fitting to a kinetic model with the extraction of all the rate constants. Determined rate constants reveal the existence of two different regimes, which are interpreted as a change in the reaction mechanism. Based on the temperature-dependent reaction behavior, it is proposed that the reaction changes from a dissociative to a molecular adsorption of oxygen on the clusters. It is found that both regimes appear for all cluster sizes, but the transition takes place at different intermediate oxides TanOx+. In general it is observed that transition occurs later for larger clusters, which is attributed to an increased cluster surface.

 Please wait while we load your content...
Please wait while we load your content...