Cage effects on conformational preference and photophysics in the host–guest complex of benzenediols with 18-Crown-6†
Abstract
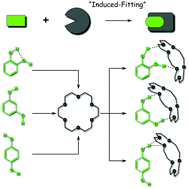
The conformational preference and modification of photophysics of benzenediols, namely hydroquinone (HQ), resorcinol (RE) and catechol (CA), upon host–guest complex formation with 18-Crown-6 (18C6) have been investigated, under supersonically jet-cooled conditions. Laser induced fluorescence (LIF) and UV–UV hole-burning spectra indicate the presence of two conformers for HQ and RE and one conformer for CA. On the other hand, the number of isomers is reduced to one in the 18C6·HQ and 18C6·RE complexes, while the 18C6·CA complex has three stable isomers. The IR spectra of the OH stretching vibration reveal that the two OH groups are H-bonded in 18C6·CA and 18C6·RE. In 18C6·RE, RE adopts the highest energy conformation in the bare form. In 18C6·HQ, the H-bonding of one OH group affects the orientation of the other OH group. The complex formation changes the photophysics of the S1 state of the benzenediols in a different manner. In our previous work, we reported a remarkable S1 lifetime elongation in 18C6·CA complexes; the S1 lifetime of CA is elongated more than 1000 times longer (8 ps → 10.3 ns) in 18C6·CA (F. Morishima et al., J. Phys. Chem. B, 2015, 119, 2557–2565), which we called the “cage effect”. In 18C6·RE, the increase of S1 lifetime is moderate: 4.0 ns (monomer) → 10.5 ns (complex). On the other hand, the S1 lifetime of HQ is shortened in 18C6·HQ: 2.6 ns (monomer) → 0.54 ns (complex). Density functional theory (DFT) calculations suggest that these behaviors are related to the S1 (1ππ*)–1πσ* energy gap, the character of the S2 state and the symmetry of benzenediol. These experimental results clearly show the potential ability of 18C6 to control the conformation and modification of the electronic structure of guest species.

 Please wait while we load your content...
Please wait while we load your content...