Extension of the interacting quantum atoms (IQA) approach to B3LYP level density functional theory (DFT)†
Abstract
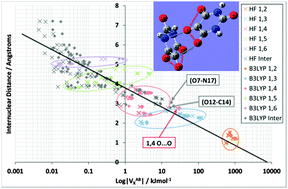
An interaction between two atoms, bonded or non-bonded, consists of interatomic contributions: electrostatic energy, exchange energy and electronic correlation energy. Together with the intra-atomic energy of an atom, these contributions are the basic components of the Interacting Quantum Atom (IQA) energy decomposition scheme. Here, we investigate IQA's proper use in conjunction with an explicit implementation of the B3LYP functional. The recovery of the total molecular energy from the IQA components is emphasised, for the first time. A systematic study of three model systems of biological relevance, N-methylacetamide (NMA), the doubly capped tripeptide GlyGlyGly and an alloxan dimer, shows the stabilization effect of B3LYP on most of the interatomic exchange energies (VABX) compared to their Hartree–Fock values. Diagrams of exchange energies versus interatomic distance show the clustering of interactions, one cluster for each 1,n (n = 1 to 6 where the atoms are separated by n − 1 bonds). The positioning of some VABX values outside their expected cluster marks interesting interactions.
- This article is part of the themed collection: Developments in Density Functional Theory

 Please wait while we load your content...
Please wait while we load your content...