Boosting water oxidation layer-by-layer†
Abstract
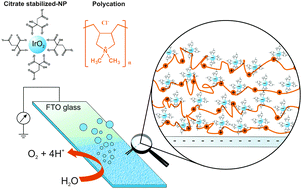
Electrocatalysis of water oxidation was achieved using fluorinated tin oxide (FTO) electrodes modified with layer-by-layer deposited films consisting of bilayers of negatively charged citrate-stabilized IrO2 NPs and positively charged poly(diallyldimethylammonium chloride) (PDDA) polymer. The IrO2 NP surface coverage can be fine-tuned by controlling the number of bilayers. The IrO2 NP films were amorphous, with the NPs therein being well-dispersed and retaining their as-synthesized shape and sizes. UV/vis spectroscopic and spectro-electrochemical studies confirmed that the total surface coverage and electrochemically addressable surface coverage of IrO2 NPs increased linearly with the number of bilayers up to 10 bilayers. The voltammetry of the modified electrode was that of hydrous iridium oxide films (HIROFs) with an observed super-Nernstian pH response of the Ir(III)/Ir(IV) and Ir(IV)–Ir(IV)/Ir(IV)–Ir(V) redox transitions and Nernstian shift of the oxygen evolution onset potential. The overpotential of the oxygen evolution reaction (OER) was essentially pH independent, varying only from 0.22 V to 0.28 V (at a current density of 0.1 mA cm−2), moving from acidic to alkaline conditions. Bulk electrolysis experiments revealed that the IrO2/PDDA films were stable and adherent under acidic and neutral conditions but degraded in alkaline solutions. Oxygen was evolved with Faradaic efficiencies approaching 100% under acidic (pH 1) and neutral (pH 7) conditions, and 88% in alkaline solutions (pH 13). This layer-by-layer approach forms the basis of future large-scale OER electrode development using ink-jet printing technology.

 Please wait while we load your content...
Please wait while we load your content...