Partitioning of mobile ions between ion exchange polymers and aqueous salt solutions: importance of counter-ion condensation†
Abstract
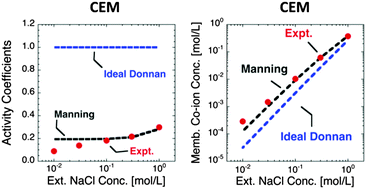
Equilibrium partitioning of ions between a membrane and a contiguous external solution strongly influences transport properties of polymeric membranes used for water purification and energy generation applications. This study presents a theoretical framework to quantitatively predict ion sorption from aqueous electrolytes (e.g., NaCl, MgCl2) into charged (i.e., ion exchange) polymers. The model was compared with experimental NaCl, MgCl2, and CaCl2 sorption data in commercial cation and anion exchange membranes. Ion sorption in charged polymers was modeled using a thermodynamic approach based on Donnan theory coupled with Manning's counter-ion condensation theory to describe non-ideal behavior of ions in the membrane. Ion activity coefficients in solution were calculated using the Pitzer model. The resulting model, with no adjustable parameters, provides remarkably good agreement with experimental values of membrane mobile salt concentration. The generality of the model was further demonstrated using literature data for ion sorption of various electrolytes in charged polymers, including HCl sorption in Nafion.

 Please wait while we load your content...
Please wait while we load your content...