Efficient electron-promoted desorption of benzene from water ice surfaces†
Abstract
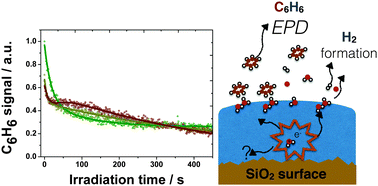
Desorption of benzene (C6H6) from solid water surfaces [compact amorphous solid water (c-ASW) and crystalline ice (CI)] during irradiation of ultrathin solid films with low energy (250–300 eV) electrons has been investigated. The observed desorption behaviour is complex but typically two desorption components, with particularly large cross-sections, were present in the observed signal. A fast component, with a cross-section up to 10−15 cm2, is attributed to desorption of isolated C6H6 molecules that are hydrogen-bonded to small clusters of water (H2O) molecules on the solid water surface. A slower component, with a cross-section of ca. 10−17 cm2, is attributed mainly to desorption from larger C6H6 islands on the solid water surface. Possible desorption mechanisms are proposed and astrophysical implications are discussed.

 Please wait while we load your content...
Please wait while we load your content...