Alkali subhalides: high-pressure stability and interplay between metallic and ionic bonds†
Abstract
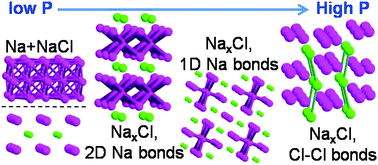
The application of high pressure (hundreds of gigapascals) to materials, besides modifying their properties, changes dramatically their reactivity. Consequently, new compounds are formed, which violate the chemical paradigms known to date. In fact, it was recently discovered (Zhang et al., Science, 2013) that sodium subchlorides (NaxCl, x > 1) become stable at high pressure. In this work, we carry out a thorough study of these compounds as well as of other alkali subhalides by means of evolutionary crystal structure prediction calculations combined with an in-depth analysis of their crystal and electronic structures. The results of our investigation are threefold. We present an updated phase diagram of NaxCl, including one new compound (Na4Cl3) and two previously undiscovered phases of Na3Cl. We demonstrate the appearance of remarkable features in the electronic structure of sodium subchlorides, such as chlorine atoms acquiring a −2 oxidation state. Most importantly, we derive a model which enables one to rationalize the stability of alkali subhalides at high pressure. The predictive ability of our model was validated by the results of crystal structure prediction calculations we carried out on alkali subhalides A3Y (A = Li, Na, K; Y = F, Cl, Br). Moreover, we show how the stability of recently reported high-pressure compounds can be rationalized on the basis of the insights gained in the present study.

 Please wait while we load your content...
Please wait while we load your content...