Intermolecular interactions and proton transfer in the hydrogen halide–superoxide anion complexes†
Abstract
The superoxide radical anion O2− is involved in many important chemical processes spanning different scientific disciplines (e.g., environmental and biological sciences). Characterizing its interaction with various substrates to help elucidate its rich chemistry may have far reaching implications. Herein, we investigate the interaction between O2− (![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) 2Πg) and the hydrogen halides (
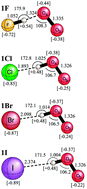
2Πg) and the hydrogen halides (![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) 1Σ) with coupled-cluster theory. In contrast to the short (1.324 Å) hydrogen bond formed between the HF and O2− monomers, a barrierless proton transfer occurs for the heavier hydrogen halides with the resulting complexes characterized as long (>1.89 Å) hydrogen bonds between halide anions and the HO2 radical. The dissociation energy with harmonic zero-point vibrational energy (ZPVE) for FH⋯O2− (
1Σ) with coupled-cluster theory. In contrast to the short (1.324 Å) hydrogen bond formed between the HF and O2− monomers, a barrierless proton transfer occurs for the heavier hydrogen halides with the resulting complexes characterized as long (>1.89 Å) hydrogen bonds between halide anions and the HO2 radical. The dissociation energy with harmonic zero-point vibrational energy (ZPVE) for FH⋯O2− (![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) 2A′′) → HF (
2A′′) → HF (![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) 1Σ) + O2− (
1Σ) + O2− (![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) 2Πg) is 31.2 kcal mol−1. The other dissociation energies with ZPVE for X−⋯HO2 (
2Πg) is 31.2 kcal mol−1. The other dissociation energies with ZPVE for X−⋯HO2 (![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) 2A′′) → X− (
2A′′) → X− (![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) 1Σ) + HO2 (
1Σ) + HO2 (![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) 2A′′) are 25.7 kcal mol−1 for X = Cl, 21.9 kcal mol−1 for X = Br, and 17.9 kcal mol−1 for X = I. Additionally, the heavier hydrogen halides can form weak halogen bonds H–X⋯O2− (
2A′′) are 25.7 kcal mol−1 for X = Cl, 21.9 kcal mol−1 for X = Br, and 17.9 kcal mol−1 for X = I. Additionally, the heavier hydrogen halides can form weak halogen bonds H–X⋯O2− (![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) 2A′′) with interaction energies including ZPVE of −2.3 kcal mol−1 for HCl, −8.3 kcal mol−1 for HBr, and −16.7 kcal mol−1 for HI.
2A′′) with interaction energies including ZPVE of −2.3 kcal mol−1 for HCl, −8.3 kcal mol−1 for HBr, and −16.7 kcal mol−1 for HI.

 Please wait while we load your content...
Please wait while we load your content...