Probing the microsolvation of a quaternary ion complex: gas phase vibrational spectroscopy of (NaSO4−)2(H2O)n=0–6, 8†
Abstract
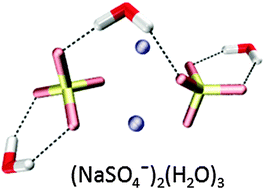
We report infrared multiple photon dissociation spectra of cryogenically-cooled (NaSO4−)2(H2O)n dianions (n = 0–6, 8) in the spectral range of the sulfate stretching and bending modes (580–1750 cm−1). Characteristic absorption bands and structural trends are identified based on a comparison to harmonic spectra of minimum-energy structures. The bare quarternary complex (NaSO4−)2 exhibits a C2h structure containing two fourfold-coordinated sodium cations in-between the two chelating sulfate dianions. Its stepwise solvation is driven by an interplay of SO42−–H2O and Na+–H2O interactions. The first water binds in a tridentate intersulfate-bridging fashion. The second and third water molecules bind to the sulfate groups at either end of the complex, which is followed by the onset of water hydrogen-bond network formation. In contrast to the binary ion pair, NaSO4−, no clear evidence for the disruption of the quaternary ion complex upon microhydration is found up to n = 8, underlining its remarkable stability and suggesting that the formation of quaternary ion complexes plays a central role in the initial stages of prenucleation in aqueous Na2SO4 solutions.

 Please wait while we load your content...
Please wait while we load your content...