Insight into the adsorption profiles of the Saprolegnia monoica chitin synthase MIT domain on POPA and POPC membranes by molecular dynamics simulation studies†
Abstract
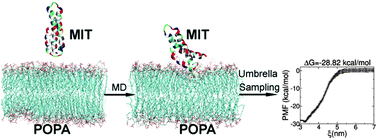
The critical role of chitin synthases in oomycete hyphal tip growth has been established. A microtubule interacting and trafficking (MIT) domain was discovered in the chitin synthases of the oomycete model organism, Saprolegnia monoica. MIT domains have been identified in diverse proteins and may play a role in intracellular trafficking. The structure of the Saprolegnia monoica chitin synthase 1 (SmChs1) MIT domain has been recently determined by our group. However, although our in vitro assay identified increased strength in interactions between the MIT domain and phosphatidic acid (PA) relative to other phospholipids including phosphatidylcholine (PC), the mechanism used by the MIT domain remains unknown. In this work, the adsorption behavior of the SmChs1 MIT domain on POPA and POPC membranes was systematically investigated by molecular dynamics simulations. Our results indicate that the MIT domain can adsorb onto the tested membranes in varying orientations. Interestingly, due to the specific interactions between MIT residues and lipid molecules, the binding affinity to the POPA membrane is much higher than that to the POPC membrane. A binding hotspot, which is critical for the adsorption of the MIT domain onto the POPA membrane, was also identified. The lower binding affinity to the POPC membrane can be attributed to the self-saturated membrane surface, which is unfavorable for hydrogen-bond and electrostatic interactions. The present study provides insight into the adsorption profile of SmChs1 and additionally has the potential to improve our understanding of other proteins containing MIT domains.

 Please wait while we load your content...
Please wait while we load your content...