Mechanisms of low temperature capture and regeneration of CO2 using diamino protic ionic liquids†
Abstract
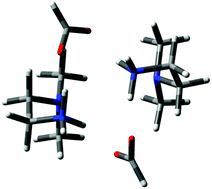
Carbon dioxide (CO2) chemical absorption and regeneration was investigated in two diamino carboxylate protic ionic liquids (PILs), dimethylethylenediamine formate (DMEDAH formate) and dimethylpropylenediamine acetate (DMPDAH acetate), using novel calorimetric techniques. The PILs under study have previously been shown to possess a CO2 absorption capacity similar to the industrial standard, 30% aqueous MEA, while requiring much lower temperatures to release the captured CO2. We show that this is in part due to the fact that the PILs exhibit enthalpies of CO2 desorption as low as 40 kJ mol−1, significantly lower than the 85 kJ mol−1 required for 30% aqueous MEA. Computational and spectroscopic analyses were used to probe the mechanism of CO2 capture, which was found to proceed via the formation of carbamate moieties on the primary amine of both DMEDAH and DMPDAH. Evidence was also found that weakly acidic counter-ions such as formate and acetate provide, unexpectedly, an additional proton acceptor site in the traditional carbamate mechanism, revealing opportunities to increase CO2 uptake capacity in the future through careful design of the anion and cation used in the PIL capture agent.

 Please wait while we load your content...
Please wait while we load your content...