Predicting long term cooperativity and specific modulators of receptor interactions in human transferrin from dynamics within a single microstate†
Abstract
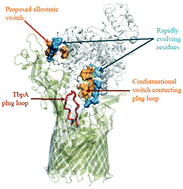
Transferrin (Tf) is an essential transport protein circulating iron in the blood and delivering it to tissues. It displays highly pH dependent cooperativity between the two lobes each carrying an iron, and forms a tight complex with the receptor during endocytosis and recycling back to the serum. We explore short-term dynamics within selected microstates of human Tf to identify functional information relevant to long-term dynamics. While the variance–covariance matrix delineates cooperativity between the domains of Tf at serum pH which is lost at endosomal pH, its decomposition does not bring about additional information. We employ perturbation-response scanning (PRS) to extract essential components that contribute to a pre-selected conformational change. Since large-scale motions may require key residues to mediate correlated motions between different regions of the protein, we use PRS to predict those involved in the conformational transitions between the iron bound and free hTf. Physiological and endosomal conditions are mimicked to identify critical residues for holo → apo and apo → holo transitions. Iron binding motions are mainly orchestrated by residues at the synergistic anion uptake sites, a finding also corroborated by additional molecular dynamics simulations where these sites are perturbed by docking the anion. Iron release is not readily accessible at serum pH, while at endosomal pH single residue perturbations on any residue encourage the large transition that involves a complex twisting of the two domains relative to each other, simultaneously opening both lobes. The pH dependent change in the dynamics is traced to the altered electrostatic potential distribution along the surface. The examination of local dynamics in the hTf–receptor pair reveals cooperativity in the quaternary structure and explains resistance to iron release in the complex. Meanwhile, the analysis of the hTf complex with a bacterial receptor that has evolved to sequester iron identifies two regions contacting rapidly evolving residues that mechanically manipulate dissociation from the pathogen.

 Please wait while we load your content...
Please wait while we load your content...