Energy transfer and formation of long-lived 3MLCT states in multimetallic complexes with extended highly conjugated bis-terpyridyl ligands†
Abstract
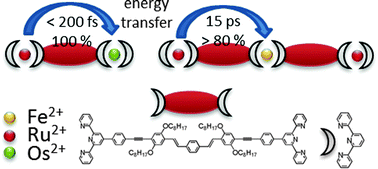
Multimetallic complexes with extended and highly conjugated bis-2,2′:6′,2′′-terpyridyl bridging ligands, which present building blocks for coordination polymers, are investigated with respect to their ability to act as light-harvesting antennae. The investigated species combine Ru(II)- with Os(II)- and Fe(II)-terpyridyl chromophores, the latter acting as energy sinks. Due to the extended conjugated system the ligands are able to prolong the lifetime of the 3MLCT states compared to unsubstituted terpyridyl species by delocalization and energetic stabilization of the 3MLCT states. This concept is applied for the first time to Fe(II) terpyridyl species and results in an exceptionally long lifetime of 23 ps for the Fe(II) 3MLCT state. While partial energy (>80%) transfer is observed between the Ru(II) and Fe(II) centers with a time-constant of 15 ps, excitation energy is transferred completely from the Ru(II) to the Os(II) center within the first 200 fs after excitation.

 Please wait while we load your content...
Please wait while we load your content...