MnCo2O4 and CoMn2O4 octahedral nanocrystals synthesized via a one-step co-precipitation process and their catalytic properties in benzyl alcohol oxidation†
Abstract
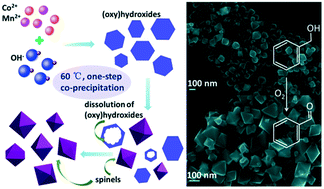
Most of the present synthetic strategies for spinel oxides rely on high-temperature processes such as hydro-/solvothermal treatments and calcination. Herein, we succeeded in synthesizing two Mn–Co mixed spinel oxides via a facile one-step co-precipitation approach. The obtained MnCo2O4 and CoMn2O4 octahedral nanocrystals were enclosed by {111} and {101} facets, respectively. Redox couples of Mn2+/Mn3+ and Co2+/Co3+ coexisted in both products. The concentration of the reactants, the manner of addition of alkali and the introduction of chloride ions could affect their crystallographic phase or particle size. A dissolution–crystallization process was deemed to be involved in their formation. Their catalytic activities were evaluated by using typical samples of MnCo2O4 (MCo1) and CoMn2O4 (CMn1) as catalysts in the aerobic oxidation of benzyl alcohol. Conversions of 82.3% and 34.1% were attained for MCo1 and CMn1, respectively. Meanwhile, a 100% selectivity for benzaldehyde was achieved for both samples during the whole reaction period. The catalytic reaction in our case proceeded through the Mars–van Krevelen mechanism. The better catalytic performance of MCo1 than that of CMn1 could be mainly ascribed to the higher content of available trivalent cations and lattice oxygens on the exposed {111} planes and their sub-layers. The high selectivity might be derived from the optimal bond strength of these two oxides as well as the mild reaction conditions we designed. Balancing activity and selectivity, our synthesized Mn–Co spinel oxides were effective and competitive in the selective oxidation of benzyl alcohol.

 Please wait while we load your content...
Please wait while we load your content...