Pyridine N-oxides as coformers in the development of drug cocrystals†
Abstract
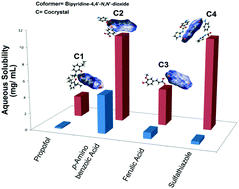
Pyridine N-oxides were synthesized via a convenient method and exploited in the preparation of drug cocrystals. Drugs associated with aqueous solubility issues such as propofol, ferulic acid, sulfathiazole, p-aminobenzoic acid etc. were used as the representative active ingredients to engineer pharmaceutical solids of a desired phase. In this study, 4,4′-bipyridine-N,N′-dioxide was picked as the cocrystal former. The types of supramolecular heterosynthons exhibited by the binary systems of N-oxide·drugs were analyzed. The formation of hydrogen bond synthons such as COOH–pyridine N-oxide, OH or NH–pyridine N-oxide was found to be robust and followed the trend observed in the Crystal Structure Database. These synthons were further correlated with the ameliorated properties of the drugs, which was supported by experiments, DFT studies and Hirshfeld surface analysis. This has been a goal in drug development.

 Please wait while we load your content...
Please wait while we load your content...