Formation mechanism of apex-truncated octahedral Cu2O microcrystal
Abstract
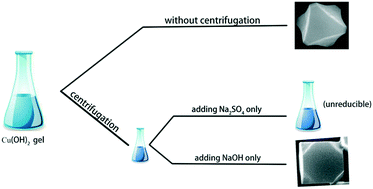
Corner-truncated octahedral Cu2O microcrystal was synthesized by a typical solution phase reduction method with CuSO4, glucose, and a small amount of NaOH. Researching into the reaction process, we found that the Cu2O microcrystal grew following the sol–gel growth model, instead of Lamer nucleation–diffusion model. The roles that OH− and SO42− played in the formation of the octahedral morphology were revealed by separating them and investigating the corresponding products, respectively. We used PVP (polyvinylpyrrolidone) in another experiment to show that the influence of the organic surfactant on the crystal habit may also be demonstrated more clearly using this method.

 Please wait while we load your content...
Please wait while we load your content...