Novel luminescent metal–organic frameworks based on rigid carboxylate ligands for highly selective sensing of Fe3+ ions†
Abstract
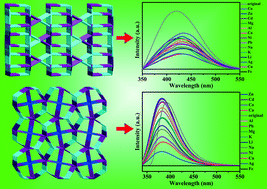
Four three-dimensional (3D) luminescent metal–organic frameworks (MOFs), Zn2(HL1)(H2O)0.8 (1), Cd2(L1)(DMF)2 (2), [Zn3(HL2)2]·[(CH3)2NH2]4 (3), and [Cd3(L2)2]·[(CH3)2NH2]6 (4) (H5L1 = 3,5-di(3′5′-dicarboxyphenyl)benzoic acid, H6L2 = 3,4-di(3,5-dicarboxyphenyl)phthalic acid, DMF = N,N-dimethylformamide), have been successfully assembled based on rigid carboxylate ligands. The four compounds have been characterized by elemental analysis, infrared (IR) spectroscopy, thermogravimetric analysis (TGA), single-crystal X-ray diffraction, powder X-ray diffraction (PXRD) and fluorescence spectroscopy. Compound 1 is a (5,5)-connected 3D framework with a {45·65} topology. Compound 2 possesses a (5,5)-connected 3D framework with a {47·63} topology. Compounds 3 and 4 display a (4,4,6)-connected 3D framework with a {42·64}2{44·611}2{44·62} topology. Remarkably, the four presented compounds show luminescence changes in the presence of metal ions. Interestingly, all of them exhibit highly selective sensing of Fe3+ ions and the luminescence is quenched in a 1.1 × 10−3 M DMF solution of Fe3+ ions. Moreover, the four compounds possess reasonable recyclability and reproducibility of luminescence after regeneration. The exceptional sensitivity and reusability of the four compounds make them excellent Fe3+ sensors in the future.

 Please wait while we load your content...
Please wait while we load your content...