Synthesis, crystal structure and luminescence studies of zinc(ii) and cadmium(ii) complexes with 6-(1H-tetrazol-5-yl)-2-naphthoic acid†
Abstract
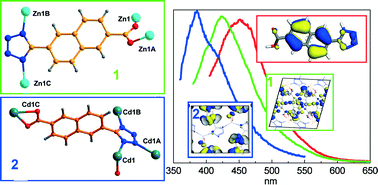
Two coordination polymers (CPs) with a novel bifunctional ligand, [ZnL]n (1) and [CdL(H2O)]n (2), (L = rigid 6-(1H-tetrazol-5-yl)-2-naphthoic acid), have been synthesized and structurally characterized, which reveal that the two constructed CPs exhibit three dimensional structures. The two CPs consist of a one-dimensional Zn-tetrazole chain and a double-layered two-dimensional Cd-tetrazole structure. In addition, IR, elemental analysis, and luminescent spectra of 1 and 2 were also examined. At room temperature, 1 exhibits an intense emission at around 421 nm upon 370 nm excitation, whereas 2 exhibits an intense emission at 381 nm upon 350 nm excitation. Through variable-temperature luminescent and quantum calculations, the blue-shift of the peaks from the ligand and 1 and 2 is ascribed to the different configurations of L caused by the different coordination environments of the metal ions.

 Please wait while we load your content...
Please wait while we load your content...