Crystal engineering urea organic acid hydrogen bonded networks with solvent inclusion properties†
Abstract
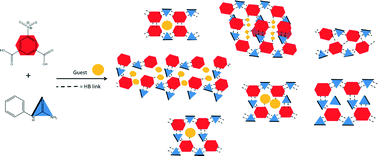
Nine hydrogen bonded networks of N-phenylurea and 5-nitroisophthalic acid, with solvent inclusion properties, have been engineered and their thermal stabilities studied. Solvent guests of methanol, ethanol, acetonitrile, acetone, THF, ethyl acetate and water have been included into the hydrogen bonded host networks in pockets and channels via interaction with a carboxylic acid group of the host. Two non-solvated N-phenylurea 5-nitroisophthalic acid complexes (NS1 2 : 1 and NS2 1 : 1) were also formed. Thermal studies of the inclusion materials revealed guest release and conversion to NS1, in all but one case, and conversion of one non-solvated form to the other (NS2 to NS1). The carboxylic acid : amide hydrogen bond synthon R22(8) was shown to be a robust synthon for network formation whilst guest molecules are suggested to have a role in templating the overall network geometry.

 Please wait while we load your content...
Please wait while we load your content...