Structural diversity of Zn(ii) coordination polymers based on bis-imidazolyl ligands and 5-R-1,3-benzenedicarboxylate and their photocatalytic properties†
Abstract
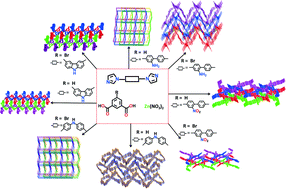
Eight Zn(II) coordination polymers with 5-R-1,3-benzenedicarboxylate (R = H, Br) ligands and bis-imidazolyl ligands, {[Zn(1,3-BDC)(abimb)]·H2O}n (1), {[Zn(5-Br-1,3-BDC)(abimb)]2·0.5H2O}n (2), [Zn(1,3-BDC)(nbimb)]n (3), [Zn(5-Br-1,3-BDC)(nbimb)]n (4), {[Zn(1,3-BDC)(bimpa)]·2H2O}n (5), {[Zn(5-Br-1,3-BDC)(bimpa)]·2H2O}n (6), [Zn(1,3-BDC)(bimcz)]n (7) and [Zn(5-Br-1,3-BDC)(bimcz)]n (8) (1,3-H2BDC = 1,3-benzenedicarboxylate; 5-Br-1,3-H2BDC = 5-bromo-1,3-benzenedicarboxylate; abimb = 2-amine-4,4′-bis(1-imidazolyl)-bibenzene; nbimb = 2-nitro-4,4′-bis(1-imidazolyl)-bibenzene; bimpa = bis-(4-imidazol-1-yl-phenyl)-amine; bimcz = 3,6-bis(1-imidazolyl)-carbazole), have been synthesized and structurally characterized by elemental analysis, IR and X-ray single crystal diffraction. 1 and 6 exhibit 4-connected 3D frameworks with the Schläfli symbol (65·8). 2, 7 and 8 display 3D polythreaded networks based on 2D wrinkled layers. 3 and 4 show 3D polycatenated arrays based on 2D puckered layers. 5 has a 3D supramolecular structure consisting of two-fold parallel interwoven layers through hydrogen bonding and π⋯π stacking interactions. The solid state photoluminescence and photocatalytic properties of 1–8 were also investigated.

 Please wait while we load your content...
Please wait while we load your content...