Fine-tuning of a thermosalient phase transition by solid solutions†
Abstract
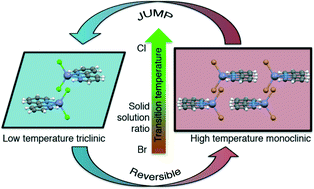
Thermosalient crystals are solids that exhibit motion at the macroscale as a consequence of a thermally induced phase transition. They represent an interesting scientific phenomenon and could be useful as actuators for the conversion of thermal energy into motion or mechanical work. The potential utilization of these miniature transducers in real-world devices requires a controllable phase transition (i.e. a predetermined temperature). While it is difficult to control these performances with a single-component molecular crystal, “tunable” properties could be accomplished by solid solutions. To verify this hypothesis, the thermosalient material [Zn(bpy)Br2] (bpy = 2,2′-bipyridine) was selected and its synthesis was performed in the presence of chloride ions. The resulting mixed crystals ([Zn(bpy)Br2xCl2(1−x)]) show that the product undergoes the expected thermosalient phase transition, and the temperature of the onset of the phase transition and the transition enthalpy depend on the Cl/Br ratio.


 Please wait while we load your content...
Please wait while we load your content...