Pressure-induced preference for solvation of 5,6-dimethylbenzimidazole†
Abstract
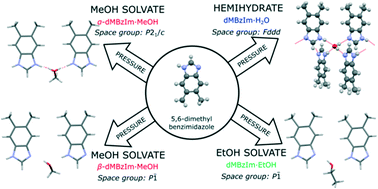
5,6-Dimethylbenzimidazole (C9H9N2) crystallizes exclusively in the unsolvated form from an aqueous solution up to 0.25 GPa and from methanol, ethanol or their mixed solutions up to 0.6 GPa. At still higher pressures, solvated crystals with water, methanol and ethanol are formed depending on the solvent used for recrystallization. The NH⋯N bonds of the neat crystals are replaced by NH⋯OH⋯N bonds in the solvates. This preference for high-pressure solvation has been rationalized in terms of changed hydrogen bonds as well as volume balance between neat and solvated compounds. The methanol solvate C9H9N2·MeOH undergoes a pressure-induced phase transition at 1.40 GPa, lowering its symmetry from monoclinic space group P21/c (phase α) to triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (high-pressure phase β) and ordering the H-atoms in hydrogen bonds NH⋯OH⋯N, disordered in phase α.
(high-pressure phase β) and ordering the H-atoms in hydrogen bonds NH⋯OH⋯N, disordered in phase α.

 Please wait while we load your content...
Please wait while we load your content...