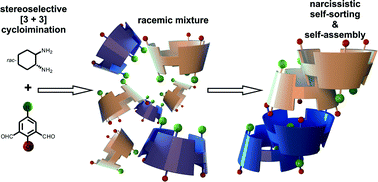
Unexpected narcissistic self-sorting at molecular and supramolecular levels in racemic chiral calixsalens†
Abstract
The [3 + 3] cyclocondensation of 2-hydroxyisophthalaldehyde derivatives with racemic trans-1,2-diaminocyclohexane results in formation of triangular vase-like hexaimines called calixsalens. Calixsalen formation is highly stereoselective, yielding racemic mixtures that contain homochiral products with absolute configuration of either all-R or all-S at their stereogenic centres. Calixsalens form tail-to-tail dimers that are assembled into higher-order structures in the solid state. The formation of these dimers is accomplished by enantioselective self-recognition of chiral calixsalen units driven by non-covalent interactions only.
- This article is part of the themed collection: Form and Function of Molecular Cups and Capsules

 Please wait while we load your content...
Please wait while we load your content...