pH-dependent formation of different coordination cages based on Co4-TC4A secondary building units and bridging ligands†
Abstract
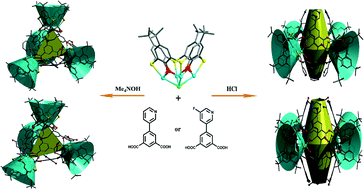
Two kinds of calixarene-based metal–organic coordination cages were obtained at different pH values despite using the same bridging ligand, either 5-(pyridin-3-yl)isophthalic acid (L1) or 5-(5-fluoropyridin-3-yl)isophthalic acid (L2), namely, {[Co4(TC4A)Cl]4(Ln)4(HCOO)4(H2O)4]}·26DMF (CIAC-117 and CIAC-119) and H4{[Co4(TC4A)Cl]4(Ln)8}·18CH3OH·33DMA (CIAC-118 and CIAC-120), where TC4A represents deprotonated p-tert-butylthiacalix[4]arene, and DMF and DMA represent N,N-dimethylformamide and N,N-dimethylacetamide, respectively. Tetrahedral coordination cages (CIAC-117 and CIAC-119) with shuttlecock-like Co4-TC4A secondary building units (SBUs) as the vertices and L1 and L2 as the three-connected linkers were prepared in the presence of tetramethylammonium hydroxide, while truncated octahedral coordination cages (CIAC-118 and CIAC-120) with L1 and L2 as the two-connected linkers were generated upon the addition of HCl. For CIAC-117, two types of activation methods, solvent exchange-heating and direct heating, were used to investigate the removal of guest molecules in the structure. N2 adsorption properties of activated samples were studied. Moreover, selective dye sorption of CIAC-120 was investigated.
- This article is part of the themed collection: Form and Function of Molecular Cups and Capsules

 Please wait while we load your content...
Please wait while we load your content...