Flow-driven morphology control in the cobalt–oxalate system†
Abstract
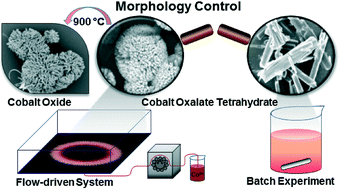
The interaction of spatial concentration gradients with chemical reactions can lead to intricate precipitate patterns. When the density difference between the reactants is sufficient, gravity current forms at the reactive interface and solid particles can sediment along radially oriented thin lines if both nucleation and crystal growth are slow. In this paper, we have studied the flow-driven Co(II)–oxalate system, by pumping cobalt nitrate solution into a thin layer of sodium oxalate solution. The formed precipitate consists of cobalt(II) oxalate tetrahydrate, identified by thermogravimetric and X-ray diffractometric analysis. This simple flow method to maintain the spatial gradients represents a controlled synthesis resulting in microstructures significantly different from those obtained in well-stirred homogeneous experiments.

 Please wait while we load your content...
Please wait while we load your content...