K(Mn,Zn)F3 mesoporous microspheres: one-pot synthesis via the nanoscale Kirkendall effect†
Abstract
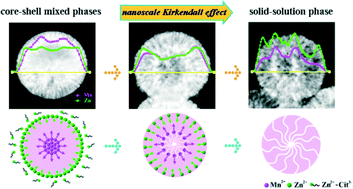
Solid-solution phase K(Mn,Zn)F3 mesoporous microspheres have been successfully prepared via a simple one-pot hydrothermal method by using citrate ions as a chelating agent. Effects of the reaction time on the phase transformation and morphological evolution of the K(Mn,Zn)F3 microspheres have been carefully investigated. The results revealed that the hierarchical mesoporous spherical structures with a solid-solution phase were formed from a core–shell structure with mixed phases via the nanoscale Kirkendall effect. It is noted that Yb3+/Er3+-codoped K(Mn,Zn)F3 microspheres exhibit intense single-band red up-conversion emission with environmental responsivity upon excitation using a 980 nm laser diode, indicating a promising potential biomedical application for drug delivery.

 Please wait while we load your content...
Please wait while we load your content...