Nanoporous lanthanide metal–organic frameworks as efficient heterogeneous catalysts for the Henry reaction†
Abstract
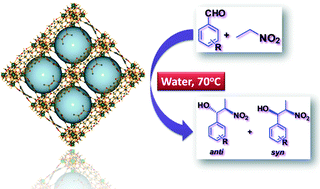
A series of self-assembled lanthanide coordination polymers formulated as [La(L1)2]n·1n(DMF)H·3n(DMF) (1), [Ce(L1)2]n·1n(DMF)H·2n(DMF) (2), [Sm(L1)2]n·1n(HCONH2)H·2n(HCONH2) (3), [La(L2)(HL2)(H2O)(DMF)2]n (4) and [Ce(L2)(HL2)(H2O)(DMF)2]n (5) [H2L1: 2-acetamidoterephthalic acid and H2L2: 2-benzamidoterephthalic acid] has been synthesized from the reactions of H2L1 or H2L2 with different lanthanide salts under hydrothermal conditions and characterized by elemental analysis, IR spectroscopy, X-ray single-crystal diffraction, powder X-ray diffraction, thermogravimetry and photoluminescence spectroscopy. X-ray diffraction analyses reveal that frameworks 1–3 have similar types of three-dimensional structures, composed of L12− ligands and trinuclear lanthanide nodes, in which two crystallographically independent lanthanide atoms are present. Frameworks 4 and 5 are isostructural, featuring a double chain-type one-dimensional coordination polymer which expands to 3D by means of H-bond interactions. These frameworks act as heterogeneous polymeric solid catalysts for the nitroaldol (Henry) reaction of different aldehydes with nitroalkanes, in water, and can be recycled without losing appreciable activity.


 Please wait while we load your content...
Please wait while we load your content...