Substrate scope for trimethyllysine hydroxylase catalysis†
Abstract
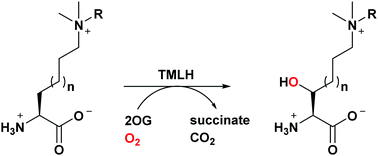
Trimethyllysine hydroxylase (TMLH) is a non-haem Fe(II) and 2-oxoglutarate dependent oxygenase that catalyses the C-3 hydroxylation of an unactivated C–H bond in L-trimethyllysine in the first step of carnitine biosynthesis. The examination of trimethyllysine analogues as substrates for human TMLH reveals that the enzyme does hydroxylate substrates other than natural L-trimethyllysine.

 Please wait while we load your content...
Please wait while we load your content...