Dehydrative condensation of carbonyls with non-acidic methylenes enabled by light: synthesis of benzofurans†
Abstract
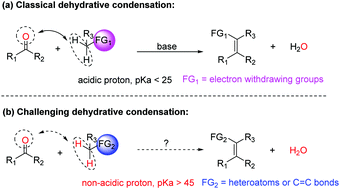
Condensation of carbonyls with non-acidic methylenes such as those adjacent to heteroatoms and allylic types to generate C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds is challenging but highly desirable. Inventing novel methods that can accomplish such condensations can enrich synthetic chemists' toolbox. Herein, we report a simple, clean, and high yielding protocol promoted by light to achieve condensation of non-acidic methylenes with carbonyls. As examples to demonstrate the power of this methodology, one class of ubiquitous and highly important heterocycles, i.e. benzofurans, were synthesized with broad functional group compatibility. Furthermore, intermolecular condensations were also briefly investigated.
C bonds is challenging but highly desirable. Inventing novel methods that can accomplish such condensations can enrich synthetic chemists' toolbox. Herein, we report a simple, clean, and high yielding protocol promoted by light to achieve condensation of non-acidic methylenes with carbonyls. As examples to demonstrate the power of this methodology, one class of ubiquitous and highly important heterocycles, i.e. benzofurans, were synthesized with broad functional group compatibility. Furthermore, intermolecular condensations were also briefly investigated.
- This article is part of the themed collection: Site-selective molecular transformations

 Please wait while we load your content...
Please wait while we load your content...