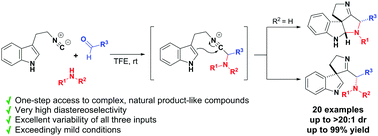
Synthesis of polycyclic spiroindolines by highly diastereoselective interrupted Ugi cascade reactions of 3-(2-isocyanoethyl)indoles†
Abstract
We report a highly diastereoselective interrupted Ugi reaction to construct a broad range of structurally congested and stereochemically complex spiroindolines from tryptamine-derived isocyanides. The reaction is facilitated by using fluorinated alcohols (TFE or HFIP) as solvents and tolerates a broad range of amines, aldehydes and 2-isocyanoethylindoles to give polycyclic products in moderate to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...