Ritter-type amination of C–H bonds at tertiary carbon centers using iodic acid as an oxidant†
Abstract
The Ritter-type amination of a tertiary C–H bond using iodic acid (HIO3) as an oxidant, in the presence of N-hydroxyphthalimide (NHPI) is reported. This operationally simple method is conducted under metal-free conditions, is scalable, and efficiently provides valuable α-tertiary amine derivatives.
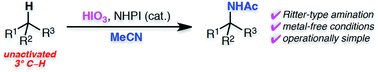

 Please wait while we load your content...
Please wait while we load your content...