Reversible water activation driven by contraction and expansion of a 12-vertex-closo-12-vertex-nido biscarborane cluster†
Abstract
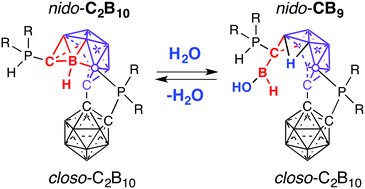
The activation of O–H bonds of water at room temperature driven by the cage-opening rearrangement of a biscarborane-based cluster is reported. The reaction of the 12-vertex-closo-12-vertex-nido biscarborane cluster with water led to the quantitative and selective two-vertex decapitation of a carborane cluster and formation of the pendent boronic acid hydride B(H)(OH) group. Remarkably, this transformation can be quantitatively reversed with the release of water and re-formation of the starting biscarborane cage. The flexibility of cage decapitation/expansion and its influence on the reactivity of an exohedral substituent represent a new approach to cluster-induced organic transformations.


 Please wait while we load your content...
Please wait while we load your content...