Hypoiodite-catalysed oxidative cyclisation of Michael adducts of chalcones with 1,3-dicarbonyl compounds: a facile and versatile approach to substituted furans and cyclopropanes†
Abstract
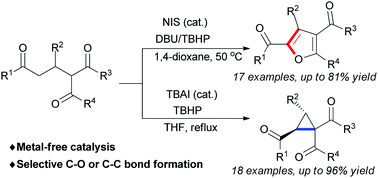
Through hypoiodite catalysis, oxidative cyclisation of Michael adducts of chalcones with 1,3-dicarbonyl compounds for divergent synthesis of either furans or cyclopropanes is developed. The selective synthesis of major products is achieved depending on the use of different reaction conditions or substrates.


 Please wait while we load your content...
Please wait while we load your content...