Aza-Morita–Baylis–Hillman reactions catalyzed by a cyclopropenylidene†
Abstract
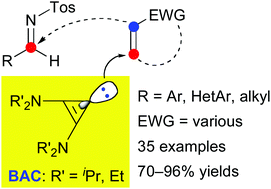
Catalysis using a bis(dialkylamino)cyclopropenylidene (BAC) has been developed, which relies on a formal umpolung activation of Michael acceptor pro-nucleophiles. Various aza-Morita–Baylis–Hillman reactions between aromatic, heteroaromatic, or aliphatic imines and acyclic or cyclic α,β-unsaturated ketones and carboxylic acid derivatives have been catalyzed by a BAC under mild conditions. Functionalities such as unprotected amino and hydroxy groups have been tolerated. The catalyst loading was decreased to 1 mol% without loss of activity. The BAC catalyst was shown to be substantially more active than a cyclic (alkyl)(amino) carbene (CAAC), N-heterocyclic carbenes (NHCs), and P- or N-centered Lewis bases.

 Please wait while we load your content...
Please wait while we load your content...