Mechanism and stereoselectivity of zeolite-catalysed sugar isomerisation in alcohols†
Abstract
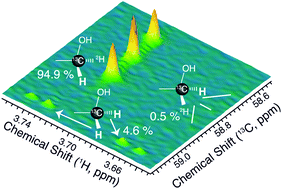
Glucose isomerisation to fructose can occur by different pathways and the mechanism of zeolite-catalysed glucose isomerisation in methanol has remained incompletely understood. Herein, the mechanism is studied using an 1H–13C HSQC NMR assay resolving different fructose isotopomers. We find that zeolite-catalysed glucose isomerisation proceeds predominantly via a hydride shift into the pro-R position of fructose, thus resembling the stereoselectivity of the enzymatic isomerisation process.


 Please wait while we load your content...
Please wait while we load your content...