Synthesis and fluxional behaviour of novel chloroborole dimers†
Abstract
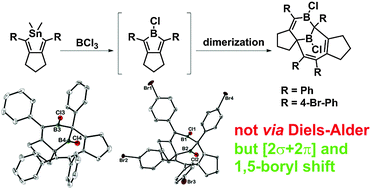
The (B-Cl)-chloroboroles 2-chloro-1,3-di(4-R-phenyl)-2,4,5,6-tetra-hydrocyclopenta[c]borole (R = H, Br) undergo a novel dimerisation process in CH2Cl2 solution. The resulting unsymmetric dimers are highly fluxional in solution via reversible enantiomerisation through an intermediate with mirror symmetry. DFT calculations suggest an unusual dimerisation mechanism and provide insight into the dynamics of the dimers.


 Please wait while we load your content...
Please wait while we load your content...