The synthesis of substituted phosphathiahelicenes via regioselective bromination of a preformed helical scaffold: a new approach to modular ligands for enantioselective gold-catalysis†
Abstract
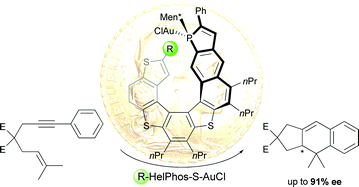
Substituted phosphathiahelicenes have been prepared via a straightforward two-step procedure involving the regioselective bromination of a preformed helical scaffold, followed by palladium catalyzed coupling reactions. The new helicenes have been used as ligands in gold(I)-catalyzed [4+2] cyclizations of 1,6-enynes. The resulting dihydro-cyclopenta[b]naphthalene derivative was obtained in excellent yields and with up to 91% ee.

 Please wait while we load your content...
Please wait while we load your content...