Direct NHC-catalysed redox amidation using CO2 for traceless masking of amine nucleophiles†
Abstract
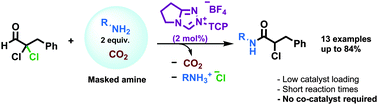
The N-heterocyclic carbene (NHC)-catalysed redox amidation reaction is poorly developed and usually requires catalytic co-additives for electron-rich amine nucleophiles. We report a masking strategy (using CO2) that couples release of the free amine nucleophile to catalytic turnover, and in doing so, enables direct catalytic redox amidation of electron-rich amines.

 Please wait while we load your content...
Please wait while we load your content...