Synthesis of 2-BMIDA 6,5-bicyclic heterocycles by Cu(i)/Pd(0)/Cu(ii) cascade catalysis of 2-iodoaniline/phenols†
Abstract
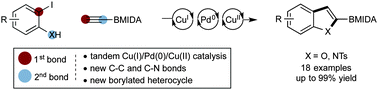
A one-pot cascade reaction for the synthesis of 2-BMIDA 6,5-bicyclic heterocycles has been developed using Cu(I)/Pd(0)/Cu(II) catalysis. 2-Iodoanilines and phenols undergo a Cu(I)/Pd(0)-catalyzed Sonogashira reaction with ethynyl BMIDA followed by in situ Cu(II)-catalyzed 5-endo-dig cyclization to generate heterocyclic scaffolds with a BMIDA functional group in the 2-position. The method provides efficient access to borylated indoles, benzofurans, and aza-derivatives, which can be difficult to access through alternative methods.

 Please wait while we load your content...
Please wait while we load your content...