Palladium(ii)-catalyzed C–C and C–O bond formation for the synthesis of C1-benzoyl isoquinolines from isoquinoline N-oxides and nitroalkenes†
Abstract
C1-Benzoyl isoquinolines can be generated via a palladium(II)-catalyzed C–C and C–O coupling of isoquinoline N-oxides with aromatic nitroalkenes. The reaction proceeds through remote C–H bond activation and subsequent intramolecular oxygen atom transfer (OAT). In this reaction, the N–O bond was designed as a directing group in the C–H bond activation as well as the source of an oxygen atom.
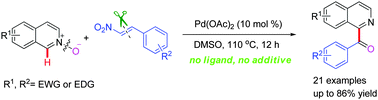

 Please wait while we load your content...
Please wait while we load your content...