Synthesis of 1,2,3,4-tetrasubstituted naphthalenes through a cascade reaction triggered by silyl acetal activation†
Abstract
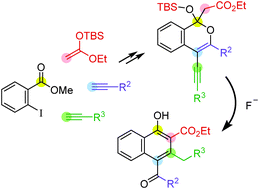
1,2,3,4-Tetrasubstituted naphthalenes bearing four different substituents were synthesized in a regioselective manner through a fluoride-induced cascade reaction of lactol silyl ethers, which could be easily prepared from 4-alkynylisocoumarins and ketene silyl acetal.

 Please wait while we load your content...
Please wait while we load your content...